How AI-Designed CRISPR Revolutionizes Gene Editing
How AI-Designed CRISPR Is Revolutionizing the Future of Medicine
For the first time, evolution is no longer the primary architect of life’s code. Discover OpenCRISPR-1, the first fully functional gene editor conceived not by nature, but by artificial intelligence. This isn’t just an optimization—it’s a paradigm shift. We’re breaking down how this AI-designed CRISPR system achieves unprecedented precision with 95% fewer errors, why its open-source release is a game-changer for global medicine, and what it means for a future where machines write the code that could cure cancer, eradicate genetic diseases, and redefine humanity itself.
Table of Contents
For decades, biology has been a science of discovery, where we learned the rules of life written by evolution. Today, we turn the page. The field of genetic engineering is undergoing its most profound transformation, moving from reading nature’s code to writing it with artificial intelligence.
In a landmark 2024 breakthrough, scientists at Profluent Bio unveiled OpenCRISPR-1—the world’s first fully functional gene-editing system designed from scratch by AI. This isn’t merely an incremental improvement on existing tools like CRISPR-Cas9. By training large language models on millions of microbial genomes, the AI generated a completely novel CRISPR system, engineered for unprecedented accuracy and efficiency.
This article delves into how AI-designed CRISPR is revolutionizing biotechnology. We will explore the creation of OpenCRISPR-1, its staggering potential to slash errors and costs, and the emerging applications from cancer therapies to climate-resistant crops. We will also confront the critical ethical questions that arise when machines hold the pencil for rewriting the code of life. This is more than a new tool; it is the dawn of a new era in precision gene editing.
The Breakthrough: How AI Engineered a New CRISPR System
The pivotal moment arrived in April 2024, when the Berkeley-based startup Profluent Bio publicly unveiled OpenCRISPR-1. This was not just another iteration of CRISPR-Cas9; it was the first fully functional gene-editing tool entirely conceived and optimized by artificial intelligence. While traditional systems like Cas9 were discovered in nature (bacteria), OpenCRISPR-1 was synthetically architected by algorithms, marking a historic departure from biological evolution as the sole source of our molecular tools.
The underlying technology is a generative AI model, trained on a massive dataset of over 5.1 million CRISPR-related protein sequences. By learning the deep grammatical rules of protein function from this “textbook of life,” the AI could then generate novel, high-performing gene editors that nature has never produced.
Why OpenCRISPR-1 Is a Paradigm Shift
This AI-driven approach to protein design translates into tangible, game-changing advantages that address the core limitations of previous gene-editing technologies.
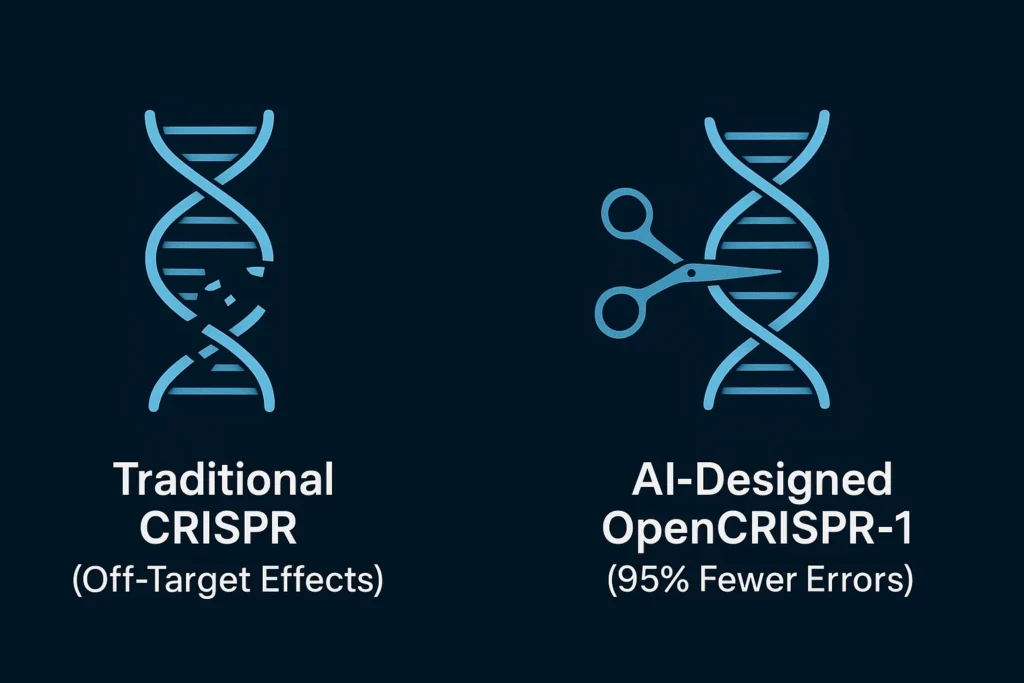
- Unprecedented Precision: The AI-optimized enzymes in OpenCRISPR-1 have demonstrated a dramatic reduction in “off-target effects”—a major hurdle where editors cut DNA in the wrong locations. Early data shows a potential reduction of errors by up to 95%, paving the way for safer human therapies.
- Open-Source Accessibility: In a move that accelerates global innovation, Profluent has released OpenCRISPR-1 as an open-source tool. This allows researchers worldwide to freely experiment, validate, and build upon this technology without restrictive licensing, rapidly expanding its potential applications. Thus, the AI-designed CRISPR system is free to use.
- Radical Cost Efficiency: The exorbitant price tag of current gene therapies (like Casgevy at $2.2 million per patient) is a significant barrier. AI-designed CRISPR systems streamline development and could drastically lower production costs, democratizing access to curative genetic treatments.
How It Works: Generative AI as the Architect of Life
The creation of OpenCRISPR-1 moves beyond simple automation; it represents a fundamental shift to generative biology. The core analogy—”ChatGPT for DNA“—is powerful because it’s accurate. Just as a large language model (LLM) learns the patterns of human language from vast text corpora, Profluent’s AI was trained on the “language of life”: a massive dataset of CRISPR protein sequences and biological contexts.
This builds upon a foundational precedent: DeepMind’s AlphaFold, which revolutionized medicine by solving the protein folding problem. Where AlphaFold gave us the ability to read the 3D structure of proteins, this new wave of generative AI gives us the tools to write entirely new ones. This allows it to generate not just text, but functional, novel gene-editing systems from first principles.
The process is a rigorous, three-stage pipeline that merges computational power with biological validation:
- Training on the Code of Life: The generative AI model, based on a refined protein language model, was trained on an immense database—the CRISPR-Cas Atlas—containing millions of CRISPR operons. It didn’t just memorize sequences; it learned the underlying “grammar” and “syntax” that govern how proteins fold, function, and interact with DNA.
- De Novo Generation: Leveraging this learned knowledge, the AI then generates completely new, optimized protein designs. It was prompted to create molecules that maintain the core function of CRISPR (DNA cutting) but are improved—smaller for delivery, more precise, and more efficient. This is how OpenCRISPR-1, a protein nearly 400 mutations away from SpCas9, was born.
- Experimental Validation: The AI’s digital blueprints are then synthesized and rigorously tested in human cells. This critical step moves from in silico prediction to in vitro reality, confirming that the AI-designed CRISPR or other editors perform their intended function with high accuracy and significantly reduced off-target effects.
From Lab to Life: The Transformative Applications
This AI-designed CRISPR pipeline is a platform technology, meaning its potential applications span across medicine, agriculture, and basic science. It could lead to breakthroughs in:
- Oncology: Engineering smarter CAR-T cells capable of targeting and infiltrating solid tumors, a major challenge in current cancer immunotherapy.
- Infectious Diseases: Developing strategies to excise persistent viral DNA, such as eradicating the HIV reservoir from a patient’s genome, potentially leading to a functional cure.
- Rare Genetic Disorders: Creating bespoke editors for thousands of monogenic diseases with a level of precision and safety previously unattainable.
- Climate-Resilient Agriculture: Designing crops that can withstand extreme weather, pests, and drought, securing the food supply in a changing climate.
The Future of AI-CRISPR: The Next Wave of Genetic Medicine
The successful creation of OpenCRISPR-1 is not the culmination of this revolution, but rather its starting pistol. It proves that generative AI can design functional biological machinery, establishing a new pipeline for discovery that is already accelerating the next wave of innovation. The future points toward more precise, safer, and highly personalized genetic interventions, moving from broad tools to bespoke cures.
Here’s what the next generation of AI-powered gene editing holds:
- Next-Generation Delivery: The challenge of delivering CRISPR into the human body is being solved with sophisticated methods like in vivo editing via lipid nanoparticles (LNPs). Therapies in development, such as NTLA-2001 for ATTR cardiomyopathy, are designed to be administered directly into the bloodstream, where these nanoparticles travel to the liver or target tissues to perform their edits—eliminating the need for complex ex vivo cell manipulation.
- Advanced Editing Modalities: Beyond cutting DNA, AI is now designing more subtle editors. Prime editing and base editing (as seen in therapies like VERVE-102 for lowering cholesterol) allow for single-letter changes in the genetic code without causing double-strand breaks. This “search-and-replace” functionality drastically improves safety and expands the range of correctable mutations.
- The AI-Accelerated Clinical Pipeline: From lab to patient, AI will streamline every step. AI-powered pipelines will not only design the editors but also predict their clinical outcomes, optimize clinical trial design, and identify potential side effects early. This integrated approach has the potential to fast-track FDA approvals and slash development timelines for new genetic medicines from decades to years.
- Fully Automated Design Cycles: We are moving toward a future of closed-loop systems where AI designs thousands of editors, robotic labs test them, and the resulting data automatically feeds back to refine the AI model. This creates a virtuous cycle of rapid innovation, compressing years of research into months.
Ethical Dilemmas: Navigating the New Frontier of AI-Designed Life
The power to rewrite the code of life with AI-driven precision forces a critical confrontation with questions we have never had to answer before. As we transition from discovering biology to programming it, the line between groundbreaking therapy and profound ethical risk becomes increasingly blurred. The creation of OpenCRISPR-1 isn’t just a scientific milestone; it is a societal challenge that demands proactive governance and global dialogue.
The core dilemmas we now face include:
- The Regulatory Vacuum: A pressing question is “Who governs AI-designed biologics (more precisely, AI-designed CRISPR)?” Current regulatory frameworks, like those at the FDA, are designed for reviewing specific, defined drugs and biologics, not for overseeing a generative AI that can produce a near-infinite number of new ones. We urgently need new, agile oversight bodies capable of evaluating this fusion of algorithmic accountability and biological safety before these tools are deployed in clinics.
- The Accessibility Paradox: While the open-source release of OpenCRISPR-1 aims to democratize innovation, it risks creating a new kind of divide. Will it truly bridge global health gaps, or will it widen them? The danger is a world where wealthy nations rapidly advance bespoke cures, while low-income countries lack the infrastructure to manufacture or deliver these complex therapies, turning a tool for equality into one of inequity.
- The Dual-Use Threat: The same technology that can eradicate malaria could, in theory, be misused to engineer more pathogenic strains. The dual-use nature of AI-designed CRISPR is its most alarming facet. The barrier to creating a dangerous biological agent is lowered when a malicious actor can use AI to design it, raising the specter of biosecurity risks and bioweapon threats that require international safeguards.
- Germline Editing and Unintended Consequences: The ethical firestorm around editing heritable human germline cells (sperm, eggs, embryos) is intensified by AI. The possibility of making permanent, irreversible changes to the human gene pool—with unknown long-term consequences—demands a global moratorium and consensus that has yet to be achieved.
We’re entering an era where machines write the code of life. The question isn’t ‘Can we?’ but ‘Should we?
— Dr. Jennifer Doudna, CRISPR Pioneer
🎥 Watch Our Deep Dive: “AI Just Rewrote CRISPR”
Ready to see this revolution in action? Our visual breakdown goes beyond the article to explore how AI is fundamentally reshaping our approach to genetic engineering.
💡 In This Video, You’ll Discover:
✅ How AI is Redesigning Life Itself: We move beyond the hype to explain the core technology of generative biology and how it’s being used to write genetic code.
✅ CRISPR vs. OpenCRISPR-1: A Clear Breakdown: What makes this AI-designed CRISPR tool fundamentally different from the natural CRISPR-Cas9 system discovered in bacteria?
✅ The Tangible Benefits: A clear look at why AI-designed gene editors are projected to be faster to develop, significantly safer, and more accessible due to their open-source nature.
✅ The Critical Ethical Debate: We dive deep into the central question: Should we trust AI to rewrite our DNA? We explore the arguments from both sides.
✅ The Future is Now: Investigating the real-world potential—can AI-designed CRISPR truly cure complex diseases with a single, precise edit?
This video is your essential guide to understanding not just what happened, but why it changes everything.
👉 Watch the Full Video on YouTube Now
Subscribe to @InSciLabTech for clearer breakdowns of the science shaping our future.
Final Thoughts: A Responsible Leap into the Next Biotech Era
The debut of AI-designed CRISPR systems marks a definitive paradigm shift in biotechnology. We are no longer limited to the tools nature provides; we can now engineer our own, optimized for precision and purpose. OpenCRISPR-1 offers tangible hope for curing once-untreatable diseases and solving monumental challenges in health and agriculture.
However, this immense power is inextricably linked with profound responsibility. The journey ahead requires more than scientific curiosity—it demands rigorous ethical frameworks, transparent public discourse, and forward-thinking international regulation. The goal is not just to innovate, but to innovate wisely.
At InSciLabTech, we are committed to tracking this revolution with a balanced perspective, championing the breakthroughs while critically examining the consequences as we navigate a future where machines help redefine life itself.
💬 Join the Global Conversation
The most important discussion is just beginning, and your voice is essential. We want to hear your perspective on this historic moment in science.
Should AI design life-saving tools, or is nature still the best engineer? What safeguards are most critical?
Choose your platform to share your thoughts, questions, and ideas:
- 💬 Comment Directly Below – Share your immediate reaction and engage with other readers right here on this article.
- 🎥 Continue the Debate on YouTube – Dive deeper into the visual breakdown and join a different audience perspective on our video page.
- 🌐 Start a Dedicated Discussion in Our Community – For in-depth, ongoing conversation, join the InSciLabTech community. We’re excited to see members start a new group focused on AI-Designed CRISPR to explore the ethics, science, and future of this technology together.
Join the InSciLabTech Community Now
Let’s build an informed and thoughtful conversation about the future we are creating.
❓ FAQ: AI-Designed CRISPR Breakthrough
What is AI-designed CRISPR?
AI-designed CRISPR refers to gene-editing systems where the molecular machinery (like the Cas protein) is not discovered in nature but is created from scratch by artificial intelligence. Using a technique called generative biology, an AI model trained on millions of protein sequences designs entirely new editors, like OpenCRISPR-1, that are optimized for higher precision and efficiency.
How is OpenCRISPR-1 different from traditional CRISPR-Cas9?
The key difference is the origin. CRISPR-Cas9 is a natural bacterial defense system that was adapted for use in labs. OpenCRISPR-1 is a synthetic gene editor that does not exist in nature. It was computationally designed by AI to be more precise, with early data showing a drastic reduction in off-target edits (up to 95% fewer errors) compared to first-generation tools.
Is AI-designed CRISPR safer than traditional gene editing?
The goal of AI-designed CRISPR is to be significantly safer. By reducing “off-target effects,” it minimizes the risk of accidentally cutting the wrong part of the genome. However, “safety” also depends on the application. The technology itself is powerful, and its safety profile for each specific therapeutic use will need to be rigorously validated in clinical trials, just like any new medical treatment.
What are the biggest risks of this technology?
The primary risks fall into two categories:
1. Technical Risks: Unforeseen biological consequences or incomplete safety validation could lead to unintended effects in patients.
2. Ethical & Societal Risks: These include the potential for dual-use (e.g., weaponization), the creation of genetic inequalities if access is not equitable, and the ethical dilemma of making heritable changes to the human germline.
Can I use OpenCRISPR-1 in my own research?
Yes, that’s a core part of the breakthrough. Profluent Bio has released OpenCRISPR-1 as an open-source tool, meaning it is freely available for academic and commercial researchers to use, modify, and build upon. This is intended to accelerate global innovation in genetic research.
How does this relate to other AI biology tools like AlphaFold?
Both are revolutionary AI applications in biology, but they solve different problems. AlphaFold predicts the 3D structure of existing proteins. The AI that created OpenCRISPR-1 generates the sequence for new, functional proteins that have never existed. Think of AlphaFold as a tool for reading and understanding biology, while generative AI is a tool for writing and designing it.





3 Comments